Keloids exhibit tumor-like but non-malignant transcriptional programs
Keloid disease is a classic example of a benign yet tumor-like fibroproliferative disorder. Clinically, keloids develop from abnormal wound healing, where dermal fibroblasts become persistently activated, producing excessive extracellular matrix (ECM) and invading nearby normal skin. Despite their locally aggressive growth, keloids remain strictly confined to the original injury site, showing no potential for metastasis or malignant change. This dual nature—tumor-like growth without malignancy—has long puzzled clinicians and molecular biologists alike. Here, we examine keloid fibroblasts as a model of benign tumor-like fibrosis, analyzing their transcriptional features relative to those of malignant programs.
Gene Set Enrichment Analysis (GSEA) highlights keloids as “tumor-like but benign.”

Identification of Key Downstream Pathways Driving Inflammation and Angiogenesis in Keloid Disease
Keloid disease is a chronic fibroproliferative skin disorder characterized by persistent inflammation, excessive extracellular matrix (ECM) deposition, and aberrant angiogenesis. Although numerous studies have implicated TGF-β1, IL-6, and the renin–angiotensin system (RAS) in keloid pathogenesis, the integrated signaling hierarchy that links cytokine activation to angiogenic remodeling remains poorly defined.
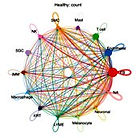
Fibroblast and endothelial enrichment with cytokine–RAS–driven inflammatory, fibrotic, and angiogenic signaling in keloid dermis
ACE inhibition suppresses cytokine-induced collagen expression in keloid fibroblasts


Molecular mechanism of the G2/M metabolic checkpoint regulation in cancer cells
Cancer cells delay/halt mitotic entry on starvation. However, we can push the cells to complete mitosis by activating Cdk1 under these conditions. I’m working on the molecular mechanism of G2/M metabolic checkpoint regulation. We`ll also study:
-
The cell’s fate once it completes the mitosis under GQ free conditions.
-
If DNA damage by UV irradiation and GQ starvation overlaps signals to halt G2/M progression.
Preliminary data(Unpublished)
Glucose (G) and glutamine (Q) starvation halt G2/M progression in HeLa cells

Abrogating the metabolic checkpoint at G2/M may cause genomic instability

UV irradiation and GQ starvation halt mitotic entry. Do they share signaling pathways?

Traction force and its regulation during cytokinesis in Dictyostelium cells
Dictyostelium cells have multiple modes of cytokinesis, including cytokinesis A, B and C. We investigated the traction stress exerted by dividing cells in the three different modes using traction force microscopy. In all cases, the traction forces were directed inward from both poles. Interestingly, the traction stress of cytokinesis A was the smallest of the three modes. Latrunculin B, an inhibitor of actin polymerization, completely diminished the traction stress of dividing cells, but blebbistatin, an inhibitor of myosin II ATPase, increased the traction stress. Myosin II is proposed to contribute to the detachment of cell body from the substratum. When the cell-substratum attachment was artificially strengthened by a poly-lysine coating, wild type cells increased their traction stress in contrast to myosin II null and other cytokinesis-deficient mutant cells, which suggests that wild type cells may increase their own power to conduct their cytokinesis. The cytokinesis-deficient mutants frequently divided unequally, whereas wild type cells divided equally. A traction stress imbalance between two daughter halves was correlated with cytokinesis failure. We discuss the regulation of cell shape changes during cell division through mechanosensing.
Though some key players have already been identified, our next project will, therefore, be on the determination of the complete picture of the mechanosensing system for proper cell division.

Cell behavior and dynamic of traction during cell adhesion and migration
The force balance between the extracellular microenvironment and the intracellular cytoskeleton is essential to a wide variety of morphogenic progresses such as division, migration and spreading. For, instance, during cell adhesion and spreading, cells generate strong traction forces on the extracellular matrix (ECM) and a mechanical feedback between traction forces and ECM compliance is thought to influence cytoskeletal organization, cell spreading and migration. Therefore, the present study has designed to explore the following three questions:
-
Morphogenic changes and traction force pattern upon cell adhesion and migration.
-
How does cell adhesion influence the traction force?
-
How do cytoskeletons controls traction forces during adhesion and migration?
Hopefully, the results of this study will open the possibility of investigating cell mechanical behaviors and cell functions and will further promote the development of cell mechanical properties as indicators of cell progression in disease states.

Traction force dynamics at collective cell migration
Collective cell movements play a vital role in morphogenesis and embryonic development and are the topic of a great deal of investigation because of their medical importance in cancer invasion, tissue regeneration and wound healing. Collective migration is defined as the ability of groups of cells to move together and simultaneously affect the behavior of one another, for example through stable or transient cell–cell connections. Thus, collective cell migration requires coordination and cooperation between migrating cells. However, cell behaviors in terms of traction force during streaming in Dictyostelium is poorly understood. Here we have an endeavor to dissect traction for dynamics during streaming in social amoeba Distyostelum discoideum.

Traction force dynamics in 3D microenvironment
Extension of traction force assays to 3D settings is important to understand migration and division of cells in the complex micro-environments found in the body.

Cell confinement at specific area
Cells in the body are physically confined by neighboring cells, tissues, and the extracellular matrix. They display great plasticity in their migration mechanisms and modulate signaling pathways and intracellular cytoskeleton and adhesion organization in response to physical constraints . Therefore, identifying physical factors driving this adaptability and discovering methods to control, manipulate, promote, or stop migration over the full range of mechanisms available to cells are vital for understanding development and morphogenesis, tuning the immune response, engineering highly structured tissues , and combating cancer metastasis. We seek to explore changes in cell migration, cytoskeletal architecture, cellular adhesions, cell signaling pathways, and gene expression that are induced by cell confinement. Our goal is to demonstrate how cell behavior in confined area can be used to improve human health.