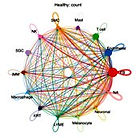
Keloids exhibit tumor-like but non-malignant transcriptional programs
Keloid disease is a classic example of a benign yet tumor-like fibroproliferative disorder. Clinically, keloids develop from abnormal wound healing, where dermal fibroblasts become persistently activated, producing excessive extracellular matrix (ECM) and invading nearby normal skin. Despite their locally aggressive growth, keloids remain strictly confined to the original injury site, showing no potential for metastasis or malignant change. This dual nature—tumor-like growth without malignancy—has long puzzled clinicians and molecular biologists alike. Here, we examine keloid fibroblasts as a model of benign tumor-like fibrosis, analyzing their transcriptional features relative to those of malignant programs.
Gene Set Enrichment Analysis (GSEA) highlights keloids as “tumor-like but benign.”

Molecular mechanism of the G2/M metabolic checkpoint regulation in cancer cells
Cancer cells delay/halt mitotic entry on starvation. However, we can push the cells to complete mitosis by activating Cdk1 under these conditions. We are currently working on the molecular mechanism of G2/M metabolic checkpoint regulation. We`ll also study:
-
The cell’s fate once it completes the mitosis under GQ free conditions.
-
If DNA damage by UV irradiation and GQ starvation overlap signals to halt G2/M progression.
By enhancing our understanding of this checkpoint, we learn more about the fundamental processes of cell division and how cancer overcomes the hostile TME.

Cells sense and respond to the physical properties of their environment and this is dependent on dynamic sub cellular systems that can generate and transduce mechanical force. Downstream integration of these signals into biochemical and genomic pathways cause observable and measurable effects on cell life. Our lab seeks to understand how cells sense and respond to mechanical forces in 2D and 3D microenvironments under different conditions during cell division and migration and to use this information to find a critical role of traction forces in a number of fundamental biological processes including cell diffentiation, angiogenesis, inflammation, wound healing, and metastasis. The social amoeba Distyostelium discoideum has been using as a model organism at our lab. We use live-cell imaging with different microscopes, biochemistry, molecular biology and computational tools to determine the functional organization of cytoskeleton with traction force.
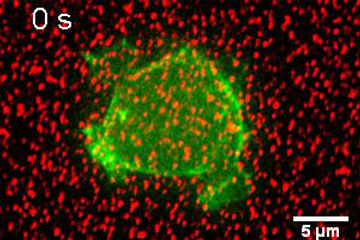
AX2 cell expressing GFP-lifeact. Beads (Force tracker) are attached on the silicone gel surface. The movie shows the movement of beads beneath the cell during division.

Visualization of traction force

Myosin II null (HS1) cell expressing GFP-lifeact
Latrunculin B, actin polymerization inhibitor was added to observe the role of actin in cell division and traction force as well. For further details, please refer to Jahan and Yumura, 2017.
Traction force and its regulation during cytikinesis in Dictyostelium cells